October 2012 saw the announcements of the Nobel Prizes in physics and chemistry, both of which were for work centring on transfers of information. While the prize-winning physics predictably involved brain bending quantum mechanics, the chemistry prize focused not on test tube reactions, but on cellular biology.
The Nobel Prize in Physics was jointly awarded to Serge Haroche, ofCollège de France and Ecole Normale Supérieure, France, and David Wineland, of National Institute of Standards and Technology (NIST) and University of Colorado Boulder, USA, “for ground-breaking experimental methods that enable measuring and manipulation of individual quantum systems.”
These experiments involve the sort of quantum weirdness described by physicist Erwin Schrodinger in 1935. By extrapolating quantum mechanics to the everyday world, he described a “ridiculous” situation in which a cat was placed in a box with a flask of poison and some radioactive material whose decay could shatter the flask. According to quantum mechanics, the cat would be both alive and dead at the same time; but of course, if anyone opened the box, they could only find a living or a dead cat.
Until recently, it had been thought difficult or impossible to observe individual atoms and sub-atomic particles that might be in two quantum states at the same time: physicists usually studied many particles at once. But sophisticated new techniques, with pioneering work by teams led by Haroche and Wineland, are helping us peer into the quantum world.
Both teams have succeeded by finding ways to trap particles.
Haroche traps light particles – photons – in a cavity between mirrors of superconducting material so reflective that a photo can remain trapped for a tenth of a second – enough time for it to travel 40,000km bouncing about in the roughly 3cm wide space. He then fires through individually prepared atoms, each of which interacts with the photon, resulting in a tiny shift in the wave motion of the atom.
Using this phase shift, Haroche can detect photons without destroying them – unlike normal detectors that convert photon energy to electrical energy. He has also monitored photons in their “cat state”, essentially oscillating both up and down at the same time, until they oscillated one way or the other.
Wineland, by contrast, keeps electrically charged atoms – ions – trapped within a vacuum in an electric field. Using a laser to encourage energy emission, his group members lower their temperatures to almost absolute zero, so they are near motionless. Here, too, he can create states like Schrodinger’s cat, with laser pulses nudging an ion into two states simultaneously.
Wineland has used the ion traps to create the world’s most accurate clocks, albeit they are so far short lived and hardly the sort of timepieces you can buy in a store. These may help with applications such as navigation. The Nobel Foundation also envisages work by Haroche and Wineland can pave the way towards creating quantum computers that could transform lives as radically as have the computers we use today.
Understanding receptors for adrenaline and more
The chemistry prize is for work that’s more readily understood, but can also have far reaching consequences. It was awarded to Robert Lefkowitz, of Howard Hughes Medical Institute and Duke University Medical Center, USA, and Brian Kobilka, of Stanford University School of Medicine, USA, “for studies of G-protein–coupled receptors.”
The origins of their work can be traced to the late 19th century, when scientists found adrenaline raised the blood pressure and heart rate, but could not discover how it worked. Adrenalin did not enter cells, so how did information get through? A mechanism involving nerves was ruled out. When a student, Lefkowitz was tasked with finding a receptor in cells producing adrenalin.
This led to a position as head of a research team in new laboratories, and Lefkowitz chose to focus on receptors for adrenalin and closely related noradrenalin. Using radioactive tagging, the team extracted receptors from living tissue. Other researchers had found what were called G-proteins within cells, and a signal from a receptor can trigger these to cause a series of reactions altering a cell’s metabolism.
Kobilka joined the team, and helped find the gene for the adrenaline receptor protein – which suggested it probably winds through the cell wall seven times. In what he later described as a “real eureka moment”, Lefkowitz realised this was the same number of spiral strings as in the light receptors others had found, and concluded there must be a family of receptors that look alike and function in the same manner.
In a new position at Stanford, Kobilka set himself the tough task of creating an image of the receptor. He needed to use X-ray crystallography, which had been successfully used for water soluble proteins. Yet the receptors are not water soluble, and Kobilka took two decades before at last obtaining an image. This was published last year, and shows a receptor at the moment it is passing a signal to a G-protein. “This image is a molecular masterpiece – the result of decades of research,” notes the Nobel Foundation.
Lefkowitz has likewise stayed at the forefront of the field, which has revealed almost a thousand genes that code for receptors, roughly half of which receive odours, a third are for hormones and other signalling substances like adrenaline, histamine, and dopamine. Some capture light; and the functions of more than a hundred are unknown.
As about half of all medications act through these receptors, they are of immense importance in medicine, and research on them can help with creating more effective drugs.
“That’s all very well,” you’re probably thinking now. “But how can I get myself a Nobel Prize?” Perhaps surprisingly, an outstanding school performance is not essential: a school report on John Gurdon, joint winner of this year’s Nobel Prize for Medicine, said his ideas about becoming a scientist were “quite ridiculous”. It might be good if you’re a man: only four women have ever won the chemistry prize. Plus: eat plenty of chocolate.
Yes, chocolate. For in more science news this week, the New England Journal of Medicine published findings that the higher a nation’s per capita chocolate consumption, the more Nobel laureates it spawns. Author Franz Messerli acknowledges this might be coincidence, but advises, “if you want a physics Nobel Prize it pretty much has got to be dark chocolate.”
Early warnings of climate change
While climate change resulting from human activities might seem a new-fangled concept, there have been on-point predictions dating back many years.…
Ignoring Science Makes Global Climate Disaster as Inevitable as Titanic Submarine Implosion
Climate change has been prominent in worldwide news this summer (2023), notably as we have just lived through the hottest week…
Have you been bullied into health? Fear, quackery and Covid
So here we are with our modern-day wonder, the internet – where even with a smartphone, you can search for and…
Never mind the antimask-o-sphere. Science shows face masks help reduce Covid spread
Just had one of those silly Twitter “conversations” with someone who had position so fixed, impossible to change with facts. Yeah,…
A Covid scrapbook: snapshots from the crazy pandemic
I’ve read accounts of the Spanish Flu, which was the last major pandemic, mainly in 1918 [so over and done with…
Highly pathogenic bird flu variants mostly evolve in intensive poultry farming
Highly pathogenic bird flu variants evolve from regular, low pathogenic, bird flus, within intensive poultry farming.
Keep Your Underpants Duck Taped and Air Clean as Covid Wild Ride Continues
We’ve learned a lot about Covid, even developing vaccines. Yet Covid remains an issue, no matter how much we might wish…
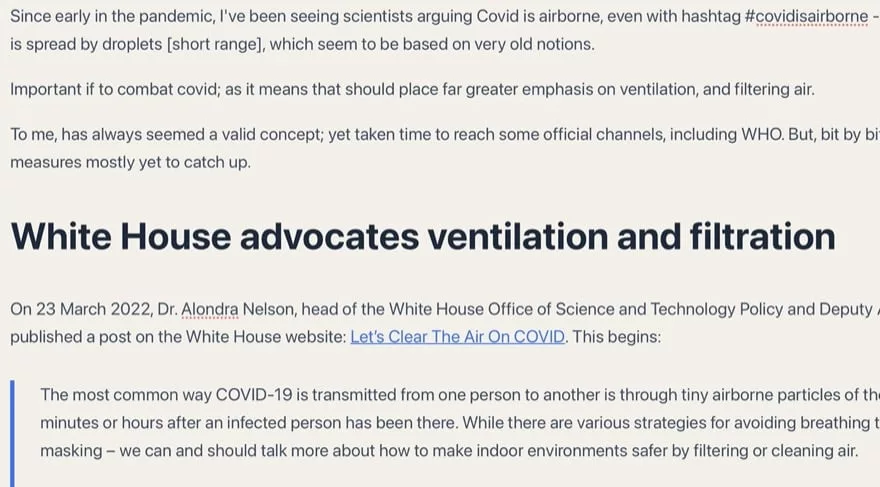
Covid is airborne so ventilation and air filtration are important
Since early in the pandemic, I’ve been seeing scientists arguing Covid is airborne, even with hashtag #covidisairborne – including to counter…
Long Covid – info and links indicating major impact
Evidence is snowballing that Long Covid is also a serious issue, even affecting people in whom the disease initially appeared mild.
Perhaps Covid arose through lab leak of tweaked bat Coronavirus
Maybe humans tweaked bat coronaviruses in gain of function experiments, inadvertently creating Covid thro lab leak.
The Covid Conundrum: Endless Lockdowns, Let It Rip … or What?
Covid is airborne, which means that much as unprotected sex is a risk for HIV, unprotected breathing might result in Covid.
Science shows Covid including Omicron is Really Not the Flu
Some of the science showing Covid including Omicron is a huge issue; and one that looks set to be with us…
“Alarmist” Covid predictions outperform Covid deniers’ soothsaying
The disinfo downplaying Covid is often from rightwing, mainly money-minded folks who perhaps don’t care too much about actual people.
Covid virulence, vaccines and variants
Science can provide some insights into what may happen with Covid, along with ways to limit its impacts.
We’re in the Covid Era for the Long Haul
We’re in this for the long haul, with the virus like a relentless, invisible foe, ready to exploit errors, slip through…
My Strange n Surprising Summer Staycation with Cellulitis
rom quick pricking by unseen marine creature, to intense fever, and hospital stay for an infection deep within the skin.
Covid virulence, vaccines and variants
[Written for South China Morning Post on 6 January 2021] In January last year, as reports were emerging of the new…
The Viral Time Bomb – Pandemic of Our Time
Guan Yi, director of the State Key Laboratory of Emerging Infectious Diseases at Hong Kong University, has extensive experience of viruses;…
From China With Fear: the Wuhan Coronavirus Won’t Kill Us All
As news of Wuhan coronavirus emerges, evolutionary biology suggests potential for a pandemic, not killing high percentage of people.
Fightback Needed as Science and Life Support System Under Attack
Environmentalism is under assault; yet this planet is the only home we have; providing our food, air, water… It’s our life…
Secret World of Hong Kong Water Supply
Hong Kong’s water supply system has been vital to its development as a “world city”.
Hong Kong Belching Buffaloes n Bubbling Paddies and the Mystery Methane Rise
Prof Euan Nisbet leads a science team to Hong Kong in quest to help find why levels of potent greenhouse gas…