Picture an atom, and you may imagine spherical electrons orbiting a nucleus packed with particles like neutrons. Only certain orbits – quantum levels – are possible. It’s a simplistic model, yet provides insights into atoms and chemical properties, and this year marks 100 years since the model was first proposed, by Danish physicist Niels Bohr.
The idea that matter comprises indivisible units dates back to Indian and Greek philosophers; one of the latter, Democritus, used the term “atomos”, meaning “uncuttable”. Yet the influential Aristotle argued that such notions were incorrect, instead favouring a theory of matter comprising four elements: fire, water, earth, and air. Quirky though it seems today, this theory prevailed, even hindering scientific progress.
It was not until around 1803 that the first useful atomic theory of matter was introduced, by British chemist-physicist John Dalton. He proposed that all matter is composed of atoms, which differ between elements and cannot be made or destroyed though can create compounds in chemical reactions.
Plum pudding model soon superseded
Almost a century later, another Briton – physicist Joseph Thomson – discovered the electron through experiments with cathode rays. This led to him proposing that atoms were made of electrons, or corpuscles as he called them, embedded in a sphere of uniform positive charge. His idea became known as the “plum pudding model”, with the electrons likened to plums within a popular pudding.
After being moribund for so long, atomic theory was now set to advance swiftly, and just five years after Thomson published his model in 1904, it was disproved by an experiment directed by a New Zealand-born scientist who became known as the father of nuclear physics, Ernest Rutherford.
The experiment involved firing positively charged alpha particles at gold foil, and seeing where they went. According to the plum pudding model, most should have passed essentially straight through, deflected by a few degrees at most. But in practice, while the majority of particles indeed passed straight on, a very small percentage were deflected at high angles, occasionally almost bouncing back in the direction they had come from.
To Rutherford, this result “was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” He concluded that each atom was mostly empty space, with a tiny but dense “central charge”, since called the nucleus.
Bohr applies quantum theory to atoms
Niels Bohr received a doctorate in physics from Copenhagen University, Denmark, and was awarded a fellowship for overseas study. As he had focused on electrons in metals, he joined Thomson’s research group in Cambridge, UK. Bohr looked forward to meeting Thomson and discussing some apparent errors with his theories, yet reportedly found him little interested in such discussions. Soon, Bohr instead spent a brief spell with Rutherford in Manchester, UK.
Based on the experiments involving gold foil, Rutherford had proposed that an atom was like a miniature solar system, with electrons circling the nucleus. But this did not fit classical theories, according to which the charged electrons should continually radiate energy and eventually spiral into the nucleus.
Returning to Denmark, Bohr began working on theories, including an exploration of whether the structure of the atom could be explained by the recently introduced quantum theory. This had arisen through German physicist Max Planck looking at materials radiating heat, and finding that the energy was always in discrete units he called quanta.
Early in 1913, a colleague drew Bohr’s attention to the Balmer series, a well known but unexplained formula that described a series of wavelengths of light in the spectrum emitted by hydrogen atoms. To Bohr, it was immediately clear that this formula dovetailed with his new theory of the atom.
Bohr postulated that an electron could follow certain stable orbits around a hydrogen nucleus, and while in these it did not give off energy. It could also fall from higher to lower levels, and energy was released during the transitions. He produced a formula that neatly agreed with the Balmer series.
During 2013, Bohr published three papers that included his new model of the atom. He had grander aims too, noting that “outlines are given of a theory of the constitution of the atoms of the elements and of the formation of molecules of chemical combinations,” yet even his atom proved hard for anyone to accept.
Theory hard to understand – but it worked!
Though Rutherford was open-minded, he considered it was “very difficult to form a physical idea” of the theory, and suggested a grave difficulty was that it implied an electron knew where it was going as it passed from one stationary state to another.
But there was no ignoring the fact that Bohr’s theory worked for the Balmer series, and even explained that a mysterious set of accompanying lines arose from helium spectra. Plum pudding notions had gone; quantum theory had become integral to the study of the atom.
Just 27 years old when he published his trilogy, Bohr became an eminent scientist, being awarded the Nobel Prize in Physics and with his name prominent among the renowned individuals at the forefront of developing quantum theory. At one conference, German genius Albert Einstein remarked, “God does not play dice”, as he disliked the uncertainties now inherent within physics. Bohr responded, “Einstein, stop telling God what to do”.
But Bohr and Einstein were mutual admirers, and in his autobiography Einstein recalled that Bohr’s atomic theory “appeared to me like a miracle”.
Atomic models swiftly evolved, with Bohr recognising that the idea of circular orbits was incorrect, and complex equations later describing probabilities of electron distributions – which only roughly correspond with orbits. These models have made it far, far harder to form a physical idea of the atom.
If you find such notions more than a little baffling, especially on a Sunday, you might take comfort in a quote from Bohr: “If anybody says he can think about quantum theory without getting giddy it merely shows that he hasn’t understood the first thing about it!”
Early warnings of climate change
While climate change resulting from human activities might seem a new-fangled concept, there have been on-point predictions dating back many years.…
Ignoring Science Makes Global Climate Disaster as Inevitable as Titanic Submarine Implosion
Climate change has been prominent in worldwide news this summer (2023), notably as we have just lived through the hottest week…
Have you been bullied into health? Fear, quackery and Covid
So here we are with our modern-day wonder, the internet – where even with a smartphone, you can search for and…
Never mind the antimask-o-sphere. Science shows face masks help reduce Covid spread
Just had one of those silly Twitter “conversations” with someone who had position so fixed, impossible to change with facts. Yeah,…
A Covid scrapbook: snapshots from the crazy pandemic
I’ve read accounts of the Spanish Flu, which was the last major pandemic, mainly in 1918 [so over and done with…
Highly pathogenic bird flu variants mostly evolve in intensive poultry farming
Highly pathogenic bird flu variants evolve from regular, low pathogenic, bird flus, within intensive poultry farming.
Keep Your Underpants Duck Taped and Air Clean as Covid Wild Ride Continues
We’ve learned a lot about Covid, even developing vaccines. Yet Covid remains an issue, no matter how much we might wish…
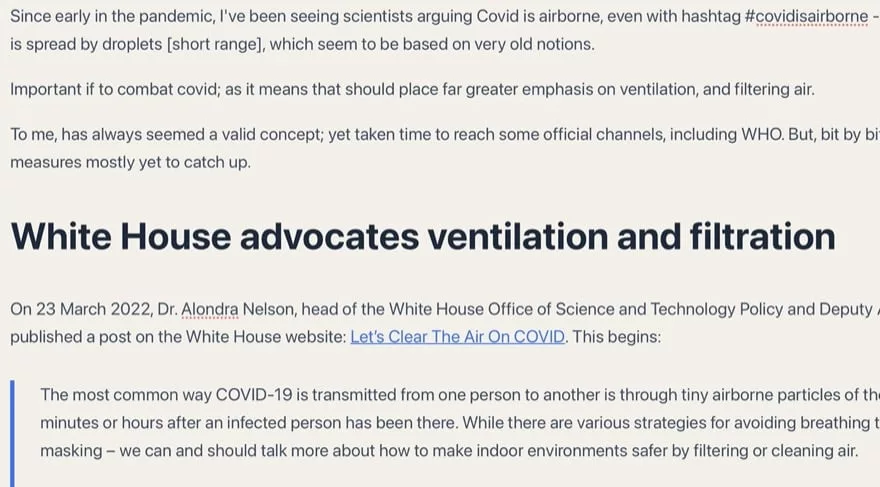
Covid is airborne so ventilation and air filtration are important
Since early in the pandemic, I’ve been seeing scientists arguing Covid is airborne, even with hashtag #covidisairborne – including to counter…
Long Covid – info and links indicating major impact
Evidence is snowballing that Long Covid is also a serious issue, even affecting people in whom the disease initially appeared mild.
Perhaps Covid arose through lab leak of tweaked bat Coronavirus
Maybe humans tweaked bat coronaviruses in gain of function experiments, inadvertently creating Covid thro lab leak.
The Covid Conundrum: Endless Lockdowns, Let It Rip … or What?
Covid is airborne, which means that much as unprotected sex is a risk for HIV, unprotected breathing might result in Covid.
Science shows Covid including Omicron is Really Not the Flu
Some of the science showing Covid including Omicron is a huge issue; and one that looks set to be with us…
“Alarmist” Covid predictions outperform Covid deniers’ soothsaying
The disinfo downplaying Covid is often from rightwing, mainly money-minded folks who perhaps don’t care too much about actual people.
Covid virulence, vaccines and variants
Science can provide some insights into what may happen with Covid, along with ways to limit its impacts.
We’re in the Covid Era for the Long Haul
We’re in this for the long haul, with the virus like a relentless, invisible foe, ready to exploit errors, slip through…
My Strange n Surprising Summer Staycation with Cellulitis
rom quick pricking by unseen marine creature, to intense fever, and hospital stay for an infection deep within the skin.
Covid virulence, vaccines and variants
[Written for South China Morning Post on 6 January 2021] In January last year, as reports were emerging of the new…
The Viral Time Bomb – Pandemic of Our Time
Guan Yi, director of the State Key Laboratory of Emerging Infectious Diseases at Hong Kong University, has extensive experience of viruses;…
From China With Fear: the Wuhan Coronavirus Won’t Kill Us All
As news of Wuhan coronavirus emerges, evolutionary biology suggests potential for a pandemic, not killing high percentage of people.
Fightback Needed as Science and Life Support System Under Attack
Environmentalism is under assault; yet this planet is the only home we have; providing our food, air, water… It’s our life…
Secret World of Hong Kong Water Supply
Hong Kong’s water supply system has been vital to its development as a “world city”.
Hong Kong Belching Buffaloes n Bubbling Paddies and the Mystery Methane Rise
Prof Euan Nisbet leads a science team to Hong Kong in quest to help find why levels of potent greenhouse gas…



















It is a great article which
It is a great article which is very informative. Looking forward to your next great article. Tibet Tour – [no spammy URLs, thanks: martin]