In January 2013, Boeing’s new, lightweight 787 Dreamliners were grounded due to issues with two of their Lithium-ion batteries overheating, leading to potential fire risks. These were the latest well-publicised yet rare, incidents involving lithium-ion batteries – which abound in our modern lives, powering devices such as mobile phones and laptops.
Though relatively new and packing some advanced features, lithium-ion batteries operate according to the basic principles of all batteries, with chemical reactions producing electrical power.
The first known batteries may date from 250 BC-640 AD. Archaeological discoveries from modern day Iran include the “Parthian jar”, which features an iron rod surrounded by a copper cylinder. When filled with an electrolyte such as vinegar it produces a small voltage. Maybe this was used for electroplating.
While the purpose of the jar is debated, the invention of the battery is credited to Alessandro Volta (1745-1827). In 1800, he demonstrated a voltaic cell: a stack of alternating copper and zinc discs in an acidic solution, which produced an electric current. His name lives on in the unit of measure for electrical potential, voltage.
Various forms of batteries have since been developed, using metals including lead, zinc, cadmium, nickel and lithium. Lithium batteries have proven especially attractive as they produce high power relatively to their size. This is chiefly because lithium atoms are tiny – with just three protons, they are larger only than hydrogen and helium – and a high electrical potential, meaning they readily lose an electron to become ions.
In a charged lithium-ion battery, there are lithium atoms within an anode material, which is usually carbon. There’s an electrolyte containing lithium ions, and a cathode with a chemical that can accept lithium ions, to result in overall chemistry that is more stable. When an electrical circuit links the anode and cathode, electrons flow from the lithium in the anode, and lithium ions move from the carbon to the cathode.
Recharging – using outside electrical power – reverses this process.
Simple concept, yet developing lithium-ion batteries proved challenging
Though the basic concept of batteries with lithium ions seems simple, development of lithium-ion batteries proved challenging. Work on lithium batteries began in 1912, yet it wasn’t until the 1970s that lithium batteries became commercially available. Sony Corporation was first to commercialise rechargeable lithium-ion batteries in 1991.
While Volta just stacked metal discs along with electrolyte, lithium-ion batteries cannot be as simple. You surely know from using a mobile phone or laptop that these batteries can become warm or hot during use. If discharge rates become too high, they can overheat, perhaps exploding or setting fire to the electrolyte. So the batteries have safety features, including voltage-limiting devices, thermal interrupts, and vents to release any excess pressure.
Even with such features, the Dreamliner batteries experienced problems. And as each weighed 63 pounds, any internal fire could prove catastrophic on an airliner. Now, a redesigned version supposedly ensures prevents fires. “Everyone feels very comfortable with what we are doing,” Ray Conner, Boeing’s CEO of commercial aircraft, said this month. “I plan to fly on the very first flight.”
Research is underway on chemistry to enhance lithium-ion batteries. A version using a combination of lithium and sulphur shows promise, as it packs more electrical power for a given volume. A version in which lithium reacts with oxygen from the air may prove more revolutionary, and is the focus of an IBM project. Yet while the concept is appealing, the science and technology director at IBM Research, USA, told Bloomberg: “We picked the path with the biggest risks and the biggest rewards. This is a moonshot.”
Early warnings of climate change
While climate change resulting from human activities might seem a new-fangled concept, there have been on-point predictions dating back many years.…
Ignoring Science Makes Global Climate Disaster as Inevitable as Titanic Submarine Implosion
Climate change has been prominent in worldwide news this summer (2023), notably as we have just lived through the hottest week…
Have you been bullied into health? Fear, quackery and Covid
So here we are with our modern-day wonder, the internet – where even with a smartphone, you can search for and…
Never mind the antimask-o-sphere. Science shows face masks help reduce Covid spread
Just had one of those silly Twitter “conversations” with someone who had position so fixed, impossible to change with facts. Yeah,…
A Covid scrapbook: snapshots from the crazy pandemic
I’ve read accounts of the Spanish Flu, which was the last major pandemic, mainly in 1918 [so over and done with…
Highly pathogenic bird flu variants mostly evolve in intensive poultry farming
Highly pathogenic bird flu variants evolve from regular, low pathogenic, bird flus, within intensive poultry farming.
Keep Your Underpants Duck Taped and Air Clean as Covid Wild Ride Continues
We’ve learned a lot about Covid, even developing vaccines. Yet Covid remains an issue, no matter how much we might wish…
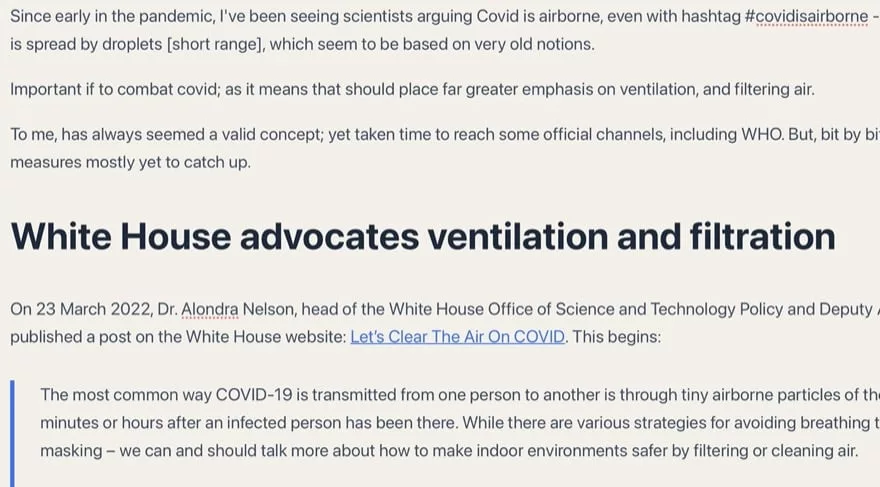
Covid is airborne so ventilation and air filtration are important
Since early in the pandemic, I’ve been seeing scientists arguing Covid is airborne, even with hashtag #covidisairborne – including to counter…
Long Covid – info and links indicating major impact
Evidence is snowballing that Long Covid is also a serious issue, even affecting people in whom the disease initially appeared mild.
Perhaps Covid arose through lab leak of tweaked bat Coronavirus
Maybe humans tweaked bat coronaviruses in gain of function experiments, inadvertently creating Covid thro lab leak.
The Covid Conundrum: Endless Lockdowns, Let It Rip … or What?
Covid is airborne, which means that much as unprotected sex is a risk for HIV, unprotected breathing might result in Covid.
Science shows Covid including Omicron is Really Not the Flu
Some of the science showing Covid including Omicron is a huge issue; and one that looks set to be with us…
“Alarmist” Covid predictions outperform Covid deniers’ soothsaying
The disinfo downplaying Covid is often from rightwing, mainly money-minded folks who perhaps don’t care too much about actual people.
Covid virulence, vaccines and variants
Science can provide some insights into what may happen with Covid, along with ways to limit its impacts.
We’re in the Covid Era for the Long Haul
We’re in this for the long haul, with the virus like a relentless, invisible foe, ready to exploit errors, slip through…
My Strange n Surprising Summer Staycation with Cellulitis
rom quick pricking by unseen marine creature, to intense fever, and hospital stay for an infection deep within the skin.
Covid virulence, vaccines and variants
[Written for South China Morning Post on 6 January 2021] In January last year, as reports were emerging of the new…
The Viral Time Bomb – Pandemic of Our Time
Guan Yi, director of the State Key Laboratory of Emerging Infectious Diseases at Hong Kong University, has extensive experience of viruses;…
From China With Fear: the Wuhan Coronavirus Won’t Kill Us All
As news of Wuhan coronavirus emerges, evolutionary biology suggests potential for a pandemic, not killing high percentage of people.
Fightback Needed as Science and Life Support System Under Attack
Environmentalism is under assault; yet this planet is the only home we have; providing our food, air, water… It’s our life…
Secret World of Hong Kong Water Supply
Hong Kong’s water supply system has been vital to its development as a “world city”.
Hong Kong Belching Buffaloes n Bubbling Paddies and the Mystery Methane Rise
Prof Euan Nisbet leads a science team to Hong Kong in quest to help find why levels of potent greenhouse gas…